Diaper Science: Super Absorbent Polymers

Diaper Science: Super Absorbent Polymers
Our most basic expectation from a diaper is that it absorbs the liquid completely and does not give it out. These features ensure that our baby’s bottom stays dry until he/she gets a toilet habit. So, how can a diaper have such a magical property? Today, we’re going to uncover the secret of a jaw-dropping magic trick to learn how to use diapers!
Tiny Dryers Inside the Diaper
Let’s say we cut the middle part of a clean diaper with the help of scissors. The resulting powdery substances are referred to as superabsorbent polymers. These magic substances have the ability to absorb liquid. We can think of it like a sponge. Although the sponges pass the liquid absorption test successfully, they fail the non-ejection test. That is, they give back the liquid they have absorbed under a little pressure. Therefore, they are not a suitable choice for diapers. Super absorbent polymers, on the other hand, do not return the liquid they absorb. In this way, the pee is prevented from coming out.

How Do Super Absorbent Polymers Absorb Liquid?
To answer this question, we need to consult the science of chemistry. The chemical name of the super absorbent polymer used in baby diapers is sodium polyacrylate. Polymers are large molecules formed by combining small molecules called monomers. Sodium polyacrylate has a long and curved structure. Our headphones, which have been waiting for a long time in our pocket, have a complex appearance, similar to the entanglement of this polymer.
The first condition of being a super absorbent polymer is to love water, that is, to be hydrophilic. The sodium portion is positive and dissolves in contact with water. The other part has a negative charge. The water molecule also consists of hydrogen and oxygen atoms with positive and negative charges. The negative parts of our long-coiled polymer are attracted by the positive parts of the water. As water continues to be added, the polymer structure begins to deteriorate and the crimped structure disappears. The surface area of the material, which takes on a flat and taut shape, also increases.

In other words, when it encounters water, the complex molecules begin to move away from each other and become flatter. However, there is a problem. The molecules are linked to each other by cross-links. Because of this condition, they are insoluble in water. Let’s rethink our headphone example. We were able to open our headphones without breaking them, and the complex headphones became flat and taut. In this way, the headset, which took up a tiny few centimeters of space in our pocket, suddenly became more than a meter long.
Superabsorbent polymers can absorb hundreds of times their own weight in liquid by increasing their surface area.
Deciphering the Magicians’ Secret!
Let’s expose the magic trick of magicians by using this information we have learned! There is a famous trick we see during juggling shows: Our professional magician takes the stage and takes a jug of water. He then shows the audience an empty (!) glass. Immediately after, he takes the water from the jug and pours it into the glass. After a short while, he shows the glass to the audience and turns the glass upside down. What is this? The glass, which should be full of water, is completely dry. The water is almost gone!
The juggler, who hears the sounds of applause after the bewildered looks of the audience, proudly ends his show. However, there is no magic.
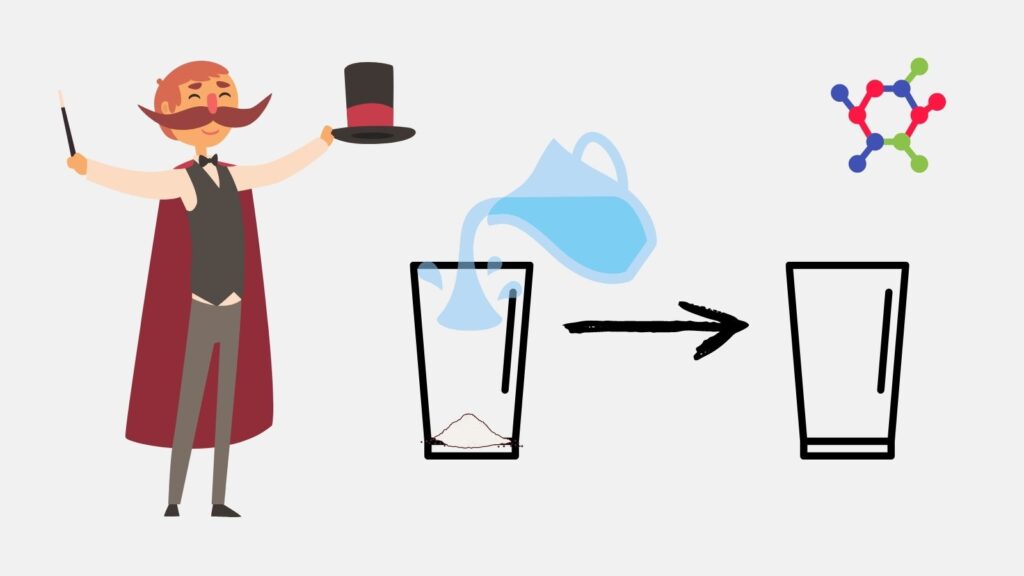
The science behind this trick is the superabsorbent polymers we just learned about. The glass shown by the juggler is not actually empty. Its base is filled with sodium polyacrylate, a white powder. The water discharged from the jug is quickly absorbed by this polymer. In this way, a completely dry appearance is formed, just like in diapers.
Behind many magic tricks such as this trick lie scientific facts and optical illusions. The closest thing to magic in the real world is science!
References and Further Readings
How Do Polymers in Diaper Absorb Liquid? TUBITAK Science Young. (2020, August 15). https://bilimgenc.tubitak.gov.tr/makale/bebek-bezsinde-polimerler-siviyi-nasil-emer.
Diapers: The Inside Story. Diapers: The Inside Story – American Chemical Society. (n.d.). https://www.acs.org/content/acs/en/education/whatischemistry/adventures-in-chemistry/experiments/diapers.html.
Encyclopædia Britannica, inc. (n.d.). Uncover the chemistry behind the absorption ability of disposable diapers. Encyclopædia Britannica. https://www.britannica.com/video/187091/diapers.
Images not cited are used through Canva Pro with a royalty payment.
The proofreading has been done by Asu Pelin Akköse and Mete Esencan.
Would you like to support us?
- For more detailed information, you can check our “Support Us!” page!







